Myelodysplastic syndrome (MDS) is a spectrum of disorders resulting from clonal expansion of hematopoietic stem cells. MDS is characterized by peripheral blood cytopenias resulting from ineffective hematopoiesis, morphologic dysplasia and a risk of disease progression to acute myeloid leukemia (AML).
The diagnosis of MDS is made by morphological review of a bone marrow aspirate by a hematopathologist. Chromosome and molecular analysis can provide important prognostic information as well as identify specific mutations or rearrangements that define specific subgroups of MDS (PMID: 37288607).
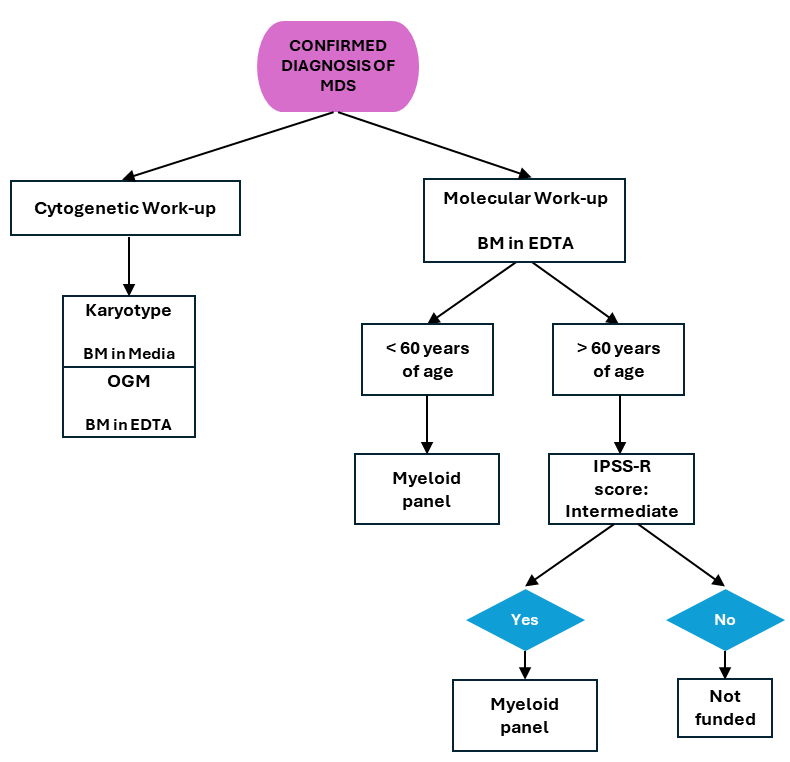
CGL Funded Criteria for the Prognostic Work-up in MDS Patients
Cytogenetic and molecular profiling are important in the standard prognostic work-up of patients with a new MDS diagnoses and in CGL the following tests which are initiated once morphological confirmation of MDS is communicated to the laboratory (receipt of a bone marrow report):
Karyotype (reflex FISH when necessary)
The analysis of 20 metaphases (25 for follow-up cases) of G-banded chromosomes. This assay requires dividing cells therefore receipt of bone marrow aspirate in media within 2-3 days from collection is vital.
This imaging technology has the ability of detecting both structural and copy number variants at a higher resolution than karyotype and is performed for all indications where a karyotype is performed (when the appropriate specimen is received – see below).
In MDS, myeloid panel is performed to further classify individuals who have intermediate-risk MDS or who are transplant eligible which includes the following
- Individuals <60 years of age with any karyotype (see flowchart below for criteria).
- Individuals 60-80 years of age in the IPSS-R intermediate risk category based on clinical and cytogenetic features (see flowchart below for criteria).
NOTE: Myeloid panel is only performed once per disease state and is not used to determine treatment response or disease progression.
CGL MDS work-up overview
TEST AND SPECIMEN REQUIREMENTS
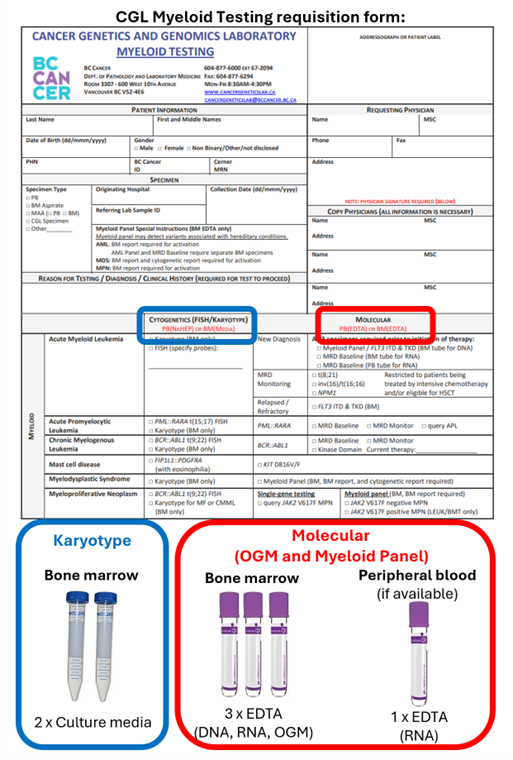
- Specimen requirements
- Karyotype – 1 mL of bone marrow aspirate in transport media (see specimen page link)
- Optical genome mapping – 0.5 mL of bone marrow aspirate in EDTA
- Myeloid panel – 0.5 mL of bone marrow aspirate in EDTA
NOTE: Two EDTA tubes are required due to different specimen preparations
- Documentation requirements
- Completed CGL Myeloid Testing requisition form
- Final bone marrow report showing features of MDS
NOTE: A bone marrow report is required to activate testing and the turnaround time starts once all documentation is received. More detailed information on sample requirements can be found here.
The following diagram can be used to assist in the packaging of specimens obtained for patients with a suspected myeloid malignancy to ensure they are received in the correct laboratory with all required specimens.
Sample packaging instructions
TURN AROUND TIME
Karyotype – 14 calendar days from receipt of a bone marrow report indication morphological evidence of MDS
Optical genome mapping – 21 calendar days from receipt of a bone marrow report indication morphological evidence of MDS
Myeloid panel – 21 calendar days from receipt of a bone marrow report indication morphological evidence of MDS
ADDITIONAL INFORMATION
International Prognostic Scoring System (IPSS)
Prognostication based on Cytogenetics results
The International Prognostic Scoring System (IPSS) or IPSS-revised (IPSS-R) is used for disease risk stratification. According to the NCCN Guidelines – MDS 2025, the IPSS should be used for initial MDS prognostication. The IPSS was developed based on the combined cytogenetic, morphologic, and clinical data from a large group of MDS cases included in previously reported prognostic studies. IPSS-R risk categories for MDS disease include: very low, low, intermediate, high, and very high. The patient’s cytogenetics test results are included in calculation of their IPSS-R score: https://ipssradvanced.mds-foundation.org/
Prognostication based on Myeloid Panel results
The International Prognostic Scoring System-molecular (IPSS-M) is a prognostic model that takes into account blood counts, marrow blasts, IPSS-R cytogenetic risk categories, and somatic variants (or DNA changes) in multiple genes and provides time estimates for overall survival, leukemia-free survival, and AML transformation (NCCN Guidelines – MDS 2025). IPSS-M risk categories for MDS disease include: very low, low, moderate low, moderate high, high, and very high. Variants identified by Myeloid Panel testing can be used to calculate the patient’s IPSS-M score: https://mds-risk-model.com/
MDS Classification
Morphological and genetic classifications of MDS exist (Table 1). Some patients with MDS also present evidence of a myeloproliferative (MPN) component defined as MDS/MPN syndrome (Table 2).
Table 1. World Health Organization (WHO) classification and International Consensus Classification (ICC) of MDS.
| WHO 2016 | WHO 2022 | ICC 2022 | General description |
| MDS, morphologically defined | |||
| MDS-SLD, MDS-MLD | MDS-LB | MDS-NOS | MDS with low blasts |
| – | MDS-hypoplastic | – | |
| MDS-EB1 | MDS-IB1 | MDS-EB | MDS with increased blasts 1 |
| MDS-EB2 | MDS-IB2 | MDS/AML | MDS with increased blasts 2 |
| – | MDS with fibrosis | – | |
| MDS, genetically defined | |||
| MDS-del(5q) | MDS-5q | MDS-del(5q) | MDS with low blasts and isolated 5q deletion |
| MDS-RS | MDS-SF3B1 | MDS-SF3B1 | MDS with low blasts and SF3B1 mutation |
| – | MDS-biTP53 | Myeloid neoplasms with mTP53 | MDS with biallelic TP53 inactivation |
Table 2. MDS/MPN overlap syndromes classification
Table 2. MDS/MPN overlap syndromes classification
| WHO 2016 | WHO 2022 | ICC 2022 |
| Chronic myelomonocytic leukemia (CMML) | CMML-1 CMML-2 | CMML-myelodysplastic (CMML-MD) CMML-myeloproliferative (CMML-MP) |
| Atypical chronic myeloid leukemia (aCML) (BCR::ABL negative) | MDS/MPN and neutrophilia | aCML |
| MDS/MPN with ring sideroblasts (RS) and thrombocytosis (T) | MDS/MPN with mSF3B1 and thrombocytosis | MDS/MPN-T-SF3B1 MDS/MPN-RS-T, NOS |
| MDS/MPN unclassifiable (MDS/MPN-U) | MDS/MPN not otherwise specified (NOS) | MDS/MPN NOS |
| – | – | MDS/MPN with i(17q) |