Myeloproliferative neoplasms (MPNs) are a group of disorders characterized by an abnormal clonal proliferation of one or more myeloid lineage cells in the peripheral blood and bone marrow. Entities categorized as MPNs in the 2016 and 2022 revisions of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues include:
| WHO (2016) | WHO (2022) | Characteristics |
| Chronic myeloid leukemia (CML) | CML | Ph chromosome, BCR::ABL1 positive |
| Essential thrombocythemia (ET) | ET | Often harbors mutations in JAK2, CALR or MPL |
| Polycythemia vera (PV) | PV | JAK2 mutated in >98% |
| Primary myelofibrosis (PMF) | PMF | Often harbors mutations in JAK2, CALR or MPL |
| Chronic neutrophilic leukemia (CNL) | Often harbors an activating CSF3R mutation | |
| Chronic eosinophilic leukemia, not otherwise specified (CEL, NOS) | ||
| MPN, unclassifiable (MPN, U) | ||
| Myelofibrosis (MF) | Can present as a de novo disorder (PMF) or it can develop from the progression of PV and ET (post-PV MF or post-ET MF) |
Mutations that drive signaling downstream of cytokine receptors involved in myeloid proliferation, such as JAK2, MPL, and CALR, are characteristic of the more common MPNs. Activating CSF3R mutations, which dysregulate the granulocyte colony-stimulating factor receptor and drive granulocytic proliferation, are often found in CNL. The identification of these recurrent mutations, or documentation of clonality through the detection of other mutations commonly seen in myeloid neoplasms, is required or supportive for the diagnosis of all MPNs (except for CML).
The diagnosis of MPN is based on hematopathology review of a bone marrow aspirate, as well as examination of the peripheral blood. Chromosomal and molecular analyses are important for defining specific MPN subtypes and for providing prognostic information.
CGL Funded Criteria for the Diagnostic and Prognostic Work-up in MPN Patients
Cytogenetic and molecular profiling is important in the standard diagnostic and prognostic work-up of patients with a new MPN diagnosis. The following tests are initiated in CGL once morphological evidence of an MPN is communicated to the laboratory (receipt of a bone marrow report).
Karyotype (with reflex FISH as needed)
Karyotype analysis of 20 metaphases (25 for follow-up cases) of G-banded chromosomes is performed for the indication of MF, CMML and CML to provide prognostic information but is not routinely performed for ET or PV. This assay requires dividing cells; therefore, receipt of bone marrow aspirate in media within 2-3 days from collection is vital.
This imaging technology is able to detect both structural and copy number variants at a higher resolution than karyotype and is performed for all indications where a karyotype is performed (when the appropriate specimen is received – see below).
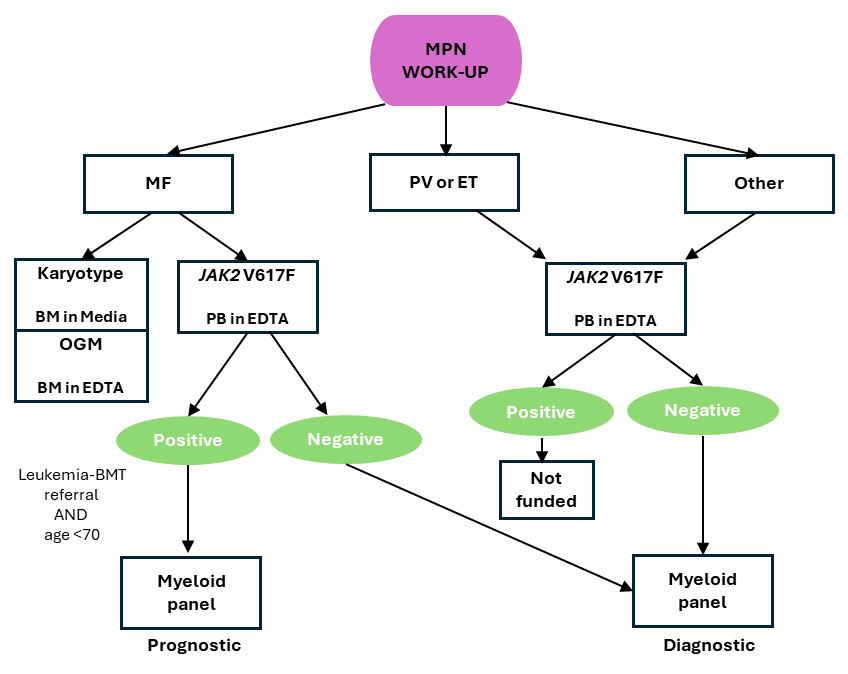
In MPN, myeloid panel is performed as a diagnostic or prognostic test as follows:
- Diagnostic testing: Individuals with suspected MPN with a negative JAK2 V617F test result.
- Prognostic testing: Individuals <70 years of age with JAK2 V617F-positive MF requiring transplant.
NOTE: Myeloid panel is only performed once per disease state and is not used to determine treatment response or disease progression.
CGL MPN work-up overview For diagnostic and monitoring workup for CML, see here.
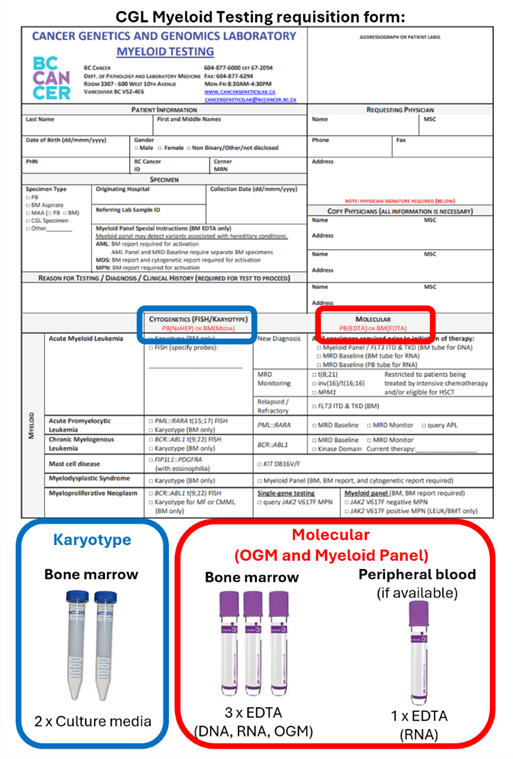
Test and specimen requirements
- Specimen requirements
- Karyotype – 1 ml of bone marrow aspirate in transport media
- Optical genome mapping – 0.5 ml of bone marrow aspirate in EDTA
- Myeloid panel – 0.5 ml of bone marrow aspirate in EDTA
NOTE: Two EDTA tubes are required due to different specimen preparations
- Documentation requirements
- Completed CGL Myeloid Testing requisition form
- Final bone marrow report showing features of MPN
NOTE: A bone marrow report is required to activate testing and the turnaround time starts once all documentation is received.
More detailed information on sample requirements can be found here.
The following diagram can be used to assist in the packaging of specimens obtained for patients with suspected myeloid malignancy to ensure they are received in the correct laboratory with all required specimens.
Sample packaging instructions
Turnaround time
Karyotype – 14 calendar days from receipt of a bone marrow report indicating morphological evidence of MPN
Optical genome mapping – 21 calendar days from receipt of a bone marrow report indicating morphological evidence of MPN
Myeloid panel – 21 calendar days from receipt of a bone marrow report indicating morphological evidence of MPN