OVERVIEW
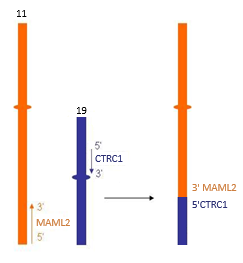
Mucoepidermoid carcinomas (MEC) typically arise within the salivary glands and is the most common salivary gland malignancy in adults. At least 80% of MEC cases harbour a t(11;19)(q21;p13.1) that results in the fusion of the 5’portion of CRTC1 (19p13.1) with the 3’ portion of MAML2 (11q21).
TEST REQUIREMENTS
- Completed CGL Cytogenetics Solid Tumour Testing requisition form
- FFPE Tumour specimen (see Specimen Guidelines, Cytogenetics FISH FFPE test type)
- An H&E stained slide with the tumour region circled, and the estimated % tumour content written in the Tumour Content field of the requisition. NOTE: A minimum of 10% tumour and at least 200 nuclei is required.
- Specimen block and/or at least one unstained slide for each probe requested.
- See our FFPE Guidelines for additional details.
TURN-AROUND TIME
Results are reported within fourteen days from receipt of specimen and completed requisition form.
RESULTS REPORTING
- Specimens are reported as Positive for a rearrangement, Negative for a rearrangement.
- Up to 80% of MEC cases will have a MAML2 rearrangement identified by this assay.
METHOD
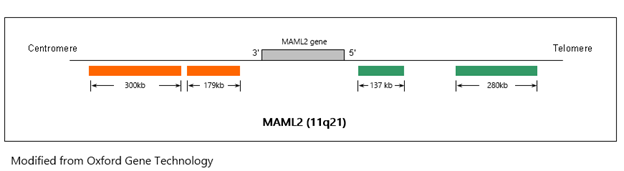
FISH analysis is performed on the provided FFPE specimen using the MAML2 (11q21) dual colour, break-apart probe (Cytocell).
REFERENCES
- Saade RE, Bell D, Garcia J, Roberts D, Weber R. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. 2016. JAMA Otolaryngol Head Neck Surg 142(3):234-40.
- Seethala RR, Chiosea SI. MAML2 Status in Mucoepidermoid Carcinoma Can No Longer Be Considered a Prognostic Marker. 2016. Am J Surg Pathol 40(8):1151-3